recurrent shingles autoimmune disease
 Shingles: Treatment, Causes, Contagious, Symptoms, Stages & Vaccine
Shingles: Treatment, Causes, Contagious, Symptoms, Stages & VaccineWarning: The NCBI website requires JavaScript to operate. University of Birmingham, University of Birmingham, Birmingham Immunology, University of Birmingham DataAbstractBackground The herpes zoster vaccine (HZ) is recommended for older adults ≥ 60 years without weakening the immune systems in the United States. It is unclear how the risk of HZ varies according to age and condition of disease for younger patients with autoimmune or inflammatory diseases (AI). We evaluate the incidence of HZ by limited age associated with AI diseases compared to adults recommended for CDC vaccination. MethodsUsing patients associated with trade and government insurance (2007-2010), we have collected seven AI cohorts: erythematous systemic lupus (SLE), intestinal inflammatory disease (IBD), rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), cothesis and two patients. We identified HZ using diagnostic codes. Specific rates of age (IR) were calculated and compared with those of IR in patients aged 60 to 69 years and without AI and diabetic conditions. Results We identified 8,395 SLE, 7,916 IBD, 50,646 RA, 2,629 PsA, 4,299 PsO, 1,019 AS, 58,934 drip, 214,631 diabetes and 330,727 periods of non-ADI registration and diabetic conditions. Higher to lower, the RIs ranged from 19.9 per 1,000 piss for SLE cohort to 6.8 for gout cohort, compared to 5.3 in patients without AI and diabetic conditions. The HZ-specific RIs for patients with RA and SLE of ≥40 were 1.5–2 times higher than those observed in healthy adults for which the vaccine is currently recommended (8.5/1000). ConclusionsSLE, IBD and RA are associated with higher HZ risks compared to older adults recommended for vaccination, suggesting that people with these conditions as young as 40 years could potentially benefit from vaccination. INTRODUCTIONHerpes Zoster (HZ), also known as shingles, is a viral disease caused by the reactivation of the latent varicela-zoster virus in cranial-nerve or dorsal-root (). HZ is characterized as a painful vesicular eruption, dermatoma and can be complicated by post-herpetic neuralgia (, ). More than one million HZ cases occur in the United States (USA) every year. Both the incidence rate (IR) and the severity of the HZ increase with the advanced age, and more than half of the people in which HZ develops are over 60 (–). The Shingles Preventive Study reported that the HZ IR for healthy people aged 60 to 69 years without autoimmune diseases was 10.8 for every 1000 years (PYs) in the non-vaccinated group (). Another randomized clinical trial reported HZ RI among individuals aged 50 to 59 years was 6.7 per 1000 PYs (). Antiviral medications approved for HZ treatment, including acyclovir, valacyclovir or famciclovir, may reduce the severity and duration of HZ, but may not prevent the pain and development of postherpetic neuralgia, which may persist for years and may be refractory to treatment(). The live HZ vaccine (Zostavax, Merck & Co., Inc.), the only vaccine licensed for HZ prevention in adults, recommended in 2006 by the United States Center for Disease Control (CDC) for adults over 60 years of age (CDC). In 2011, the Food and Drug Administration (FDA) approved the use of HZ vaccination in healthy adults aged 50 to 59 ()(). However, perhaps due to the lower absolute risk of HZ in this group, concern about the length of response and the scarcity of HZ vaccines, the CCD Advisory Committee on Immunization Practices did not recommend the adult vaccine from 50 to 59 years(). In addition to age, an individual's immune system is an essential factor in the reactivation of the varicella-zoster virus (). Studies have suggested that HZ events are several times greater among patients with AI conditions such as SLE and RA compared to patients with no autoimmune diseases or inflammatory conditions (AIR). Because the HZ vaccine is a live attenuated vaccine, its use in patients with AI conditions remains the subject of debate, although patients with AI conditions have a higher risk of HZ (, ). Despite the scarcity of data, vaccination is not recommended for patients who are on high-dose steroids or certain medicines such as biological therapies. In 2012, the American College of Rheumatology (ACR) endorsed the recommendations of the IAPA on the proper use of HZ vaccination among rheumatoid arthritis (RA) patients over 60 years of age. In 2015, the RTA reduced the recommendation of the zoster vaccine to patients of the RS 50 and more (). No other recommendations have been made on the vaccination of persons with other autoimmune or rheumatic diseases. Given the appreciable benefits of the vaccine to reduce the burden of zoster-related disease, it is unclear whether the absolute risk of HZ for younger patients with AI conditions could be high enough to justify vaccination at ages prior to the recommendation of the CDC. Therefore, we evaluate the total absolute incidence and age of HZ infection associated with different AI diseases compared to adults without AI conditions. We hypothesize that the absolute rate of HZ in younger patients with AI diseases would be comparable to or higher than the corresponding rate of HZ in healthy adults over 60 years without AI, for which vaccination against the zoster is strongly recommended. STUDY DESIGNData sources The Office of the Department of Health and Human Services (HHS) of the Assistant Secretary of Planning and Evaluation (ASPE) and the Medicare and Medicaid Service Centres (CMS) built a national multi-computer claims database (MPCD) to better support comparative effectiveness research (). With greater geographical coverage and clinical representation than single source data, the MPCD database incorporated public and private data, representing recipients with coverage of United Healthcare, Medicare or Medicaid during 2007-2010. MPCD data contains demographic and insurance coverage of patients with registration files, outpatient and outpatient services claims and prescription drugs. Study and Population Design We conducted a retrospective study using MPCD in 2007-2010. After applying inclusion and exclusion criteria, we mount 7 mutually exclusive AI cohorts, including systemic erythematous lupus (SLE), intestinal inflammatory disease (IBD), rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and gout. These AI disease cohorts were mutually exclusive of two comparison cohorts: diabetes and adults without AI or diabetic conditions. As the cohort selection diagram shown in , it is required that all patients in AI cohort or diabetes cohort disease meet the following criteria of inclusion: 1) at least two doctors diagnose specific disease that were separated between 7 and 365 days; 2) at least one full prescription or administration of specific disease treatments (–) (See diagnosis and list of medicines in ); 3) at least 1 month continuous medical coverage and pharmacist. Thus, the follow-up start date (defined as the date of the index) was the most recent date after which three conditions were met: the second diagnosis of AI disease or diabetes, the first date of prescription drugs for that condition, or the first date of the month 13 of total coverage of the health plan. To increase the homogeneity of the patient's cohorts, several exclusion criteria were also applied. Since patients with other autoimmune diseases, HIV or malignant neoplasms (excluding nonmelanoma skin cancer) may experience a higher risk of HZ, we exclude patients with medical diagnosis of these conditions. For a similar reason, patients with zoster diagnosis or antiviral prescription at any time before the index date were also excluded from the final cohort. Patients in all cohorts were followed until 31 December 2010, experienced HZ, died or lost coverage. As a small proportion of patients (As a comparative cohort, we derived a cohort of healthy patients compared to people over 20 years old, who had 13 months of medical and pharmacological coverage, and had no diagnostic code or drugs associated with any of the diseases or diabetes AI. Patients in the compared cohort without AI or diabetic conditions began their follow-up on the first date of the 13th month of full coverage and follow-up completed on 31 December 2010 or when patients experienced HZ, died, lost coverage or found any diagnostic code for an IA Herpes zoster disease result The result was the first HZ event during follow-up. We identify HZ using an International Classification of Diseases, 9th Review, Clinical Modification (ICD-9-CM) outpatient or outpatient diagnostic code (053.x). This algorithm has been previously validated and has been shown to have high sensitivity and positive predictive values (set up85%) to identify the HZ incident (, ).HZ-related FactorsWe examine factors that could confuse age associations and autoimmune diseases with HZ using baseline data. We use the current procedural terminology code (CPT) 90736 or National Drugs Codes (NDC) for the HZ vaccine to identify patients who received HZ vaccination. The age at the index date was categorized by 10-year increases from 21 to 30 years to over 70 years. The state of vaccination against HZ was dichotomous and variable time, which means that patients could enter the test as if they had not been vaccinated, and then change the state of vaccination during follow-up. The use of biologics and glucocorticoids was also defined as variable time during follow-up. For each day, we evaluate whether the patient was in biologic or not based on the days of supply or infusion after identifying the injected biologics using pharmacies and infused drugs (e.g., biological) using the Common Procedures Coding System of Part B (HCPCS) codes (, ). We classify daily glucocorticoids in three categories: none, ≤ 5 mg/day and ± 5 mg/day of prednisone (or equivalent). Other factors we examined during the baseline were: gender, comorbidities, concurrent drugs and long-term residence. Comorbidities included previous outpatient infection, pre-hospitalized infection, pre-hospitalization due to complete causes, chronic obstructive pulmonary disease, heart failure, angina and diabetes. Concurrent drugs including metotrexate, nonsteroidal anti-inflammatory drugs, drugs, hypertension, antidepressants and antihyperlipid medications. We identify the long-term care residency status of patients using algorithms previously published (). We compared the baseline characteristics of patients in nine cohorts. For each cohort, we calculate the absolute IR specific for every 1,000 person-years with 95% confidence intervals (CI), and then calculate the incidence rate (IRR) with 95% CI for each age category in each AI disease or cohort of diabetes compared to patients 60 to 69 years in the cohort without AI or diabetic conditions. Because the FDA approved the HZ vaccine for people over the age of 50, however the ACIP CDC recommendation is to vaccinate healthy patients of ages ≥ 60 (and not people of 50 to 59 years), so we derive our non-lower bond between the 10-year-old strata in all diseases., We obtained our non-inferiority margin based on the 69-year-old HZ IRs reported in the Preventive study. Therefore, we use a margin of no inferiority of 0.62, derived from a ratio of incidence [IRR] of 6.7 / 10.8 For each group of 10 years specific IRR, we classify HZ RI for each cohort as significantly higher (lower limit of 95% CI ±1.0), comparable (i.e., not lower, defined as the lower limit of 95% CI ± 0.62 but ≤ 1.0), or inconclusive (any other result), using the specific age HZ IR in healthy people 60 to 69 years. Age could confuse comparisons between cohorts if the cohort groups of the disease had different age distributions. Therefore, we calculate the standardized IR per 1,000 PYs with 95% CI using the population of the 2010 U.S. Census and as important potentially confusing factors, the effects of diabetes and glucocorticoids between SLE and RA cohorts were evaluated. A Cox regression model adjusted for age, gender, race and vaccination was used to determine the risk ratio (HR) for HZ in cohorts. To avoid adjusting to the variables in the causal pathways with AI conditions, comorbidities and concurrent use of AI medications measured during the baseline were not included in the model. As a subgroup analysis, we evaluate the extent of the benefit of HZ vaccination in patients with AI diseases. However, because few patients under 60 were vaccinated, and we had a reasonable absolute number of patients vaccinated only in the RA cohort, therefore, we only included patients over 60 years of age with RA in this additional analysis. We calculate the incidence rate of HZ by the state of vaccination of patients and the age of vaccination between patients with RA and patients without AI or diabetic conditions. Cox regression models were built to evaluate the HZ crude and adjusted risk ratio associated with the benefit of HZ vaccination in HZ incidence. We perform two adjustment levels. The initial adjustment included age, sex and race to take into account demographic differences among patients with different vaccination status. The subsequent adjustment also included the biological use of time variance and the average daily dose of oral glucocorticoids. The interaction between vaccination and the dose of glucocorticoids in a secondary analysis was also evaluated. In a sensitivity analysis, we identify HZ using a diagnostic code for ICD 9 patients alone (053.x) or an outpatient diagnosis code plus a claim for an antiviral medication within 30 days of the code, and then we repeat all the tests. All analyses were performed using SAS 9.3. The study was approved by the Institutional Review Board of the University of Alabama (IRB) and governed by a Data Use Agreement (DUA) of the data provider. RESULTSOur final study population consisted of 330,727 periods of inscription in cohort without AI or diabetic conditions, 214,631 with diabetes, 8,395 with SLE, 7,916 with IBD, 50,646 with RA, 2.629 with PSA, 4.299 with PsO, 1.019 with AS and 58,934 with drop. The selection of the final cohort for the RA is shown in , and selection criteria similar to other cohorts were applied. The baseline characteristics of the nine cohorts are presented in . More than 65% of patients in diabetes, RA and gout cohorts were over 60 years old; 90% of SLE and 78% of RA patients were women and 73% of AS patients were men. Generally, the comorbidities and reference medications were similar in the 7 AI conditions. Patients with SLE had a high proportion of kidney diseases and outpatient infections. Compared to cohort without AI or diabetic conditions, gout patients had a higher rate of diabetes, heart failure, kidney disease, hypertension medication, antihyperlipidemia medication. As expected, few patients in diabetes and AI-free cohort or diabetic conditions used oral glucocorticoids or DMARDs, unlike patients in SLE and RA cohorts, among which 40% of patients took glucocorticoids during the baseline. Table 1Patient line letters in different autoimmune diseases and comparison cohorts Subject characteristicsDisease TypeNOAID*DiabetesSLEIBDRAPSOASGoutNumber of patients328,580212,8068,3207,85850,2682,6094,2721,0115918,598Number of registration periods3301,7214,639418. Race White51.9363.2655.4374.0372.1271.8167.3270.6667 Black10.4115.6125.297.7310.534.075.957.0718.28 Asian2.682.951.750.851.712.402.364.58 Hispanic5.786.137.082.605.394.075.864.512.45 Other1.101.841.810. 701.68 per day. Based on the preset non-lower margin of a HZ IRR of 0.62, we classify the specific RRs of the age-diseas in three categories: significantly higher (red), comparable (yellow), or inconclusive or lower (without shaving). The specific HZ rate for patients with SLE, IBD and RA in their 20, 30 and 40 years was comparable or substantially higher than the corresponding rate for adults without AI ≥ 60 years (see ). Table 2Incidences of herpes zoster for every 1000 years by group of 10 years and autoimmune or cohort comparisonCohortesHealthy*DiabetesSLEIBDRAPsOASGoutIRIRIRIRIRIRIRIRIRIRIRIRIRIRIRIRIRAge group21–302.77.824.611.66.6N/A5.931–40325.329. Incidence for every 1000 years Compared to healthy older persons aged 60 to 69 years (Blue), rates were classified as significantly higher (dark red), comparable (light yellow arrow) and others (i.e. inconclusive or lower, not shaded). Based on the HZ IRs reported in the Shingles Preventive Study for the healthy general population age 60–69 of 10.8 per 1000 PY and comparing to the IR for patients aged 50–59 years of 6,7 per 1000 PY in another randomized study, selected a margin of no lowerity of 0.62( incidence ratio of 6.62 / 10.8 = 0,8 = 0.62). PsA = Psoriatic arthritis; AS = Ankylosing spondylitis; The standardized IR age for each cohort adjusted to 2010 U.S. census population (jo20 years) is presented in . Throughout the 7 AI disease cohorts, the standardized IR ranged from a height of 20.0 per 1000 years (SLE) to a low of 6.8 (gout). For diabetes, the associated age standardization rate was 8.0/1,000, and for cohort without AI or diabetic conditions, it was 5.3/1000. Standardized age IRs for female patients were numerically superior to those of male patients in all cohorts (no data shown).*between adults in use age 20SLE = Systemic erythematous lupus; IBD = inflammatory bowel disease; RA = rheumatoid arthritis; PsO = glyriasis; Pscorides = Psoritic arthritis Patients who use glucocorticoids experienced higher RIs than those patients who do not use any glucocorticoids. However, diabetes as a concurrent comorbidity with RA and SLE did not significantly elevate the HZ IR. Incidence rate based on different combinations of diabetes and dose of glucocorticoids, using RA and SLE as examples Compared to patients in cohort without AI or diabetic conditions, adjusted RH for AI and diabetes cohorts increased significantly (). After controlling age, sex, race and vaccination, HZ rates were approximately 1.5 times (diabetes) at about 3 times (SLE) higher than patients in cohort without AI or diabetic conditions. Raw and adjusted HRHCs associated with HZ vaccination among RA patients are shown in . Vaccinated patients were less likely to have later HZ events than non-vaccinated patients. Both the use of biological and oral glucocorticoids was associated with a high risk of HZ in the fully adjusted model 2, and the highest dose of glucocorticoids was associated with increased intensity with the incidence of HZ. There was no significant interaction between vaccination and the use of glucocorticoids. It was also observed that patients in the cohort of healthy patients had a significantly lower HZ rate associated with vaccination (not shown). (0,021) Model 2 adjusts all variables in the table. Non-biological DMARDs and other comorbidities in analysis were not significant. Sensitivity analysis using diagnostic codes and antiviral medications to identify cases of HZ led to similar or higher IRs and RIRs compared to the main analysis (). HZ rates in patients with IBD from 51 to 60 years or older, RA from 41 to 50 years or older, drop from 61 to 70 years or older and PsA from 71 to 85 years were significantly higher than patients aged 61 to 70 years in cohort without AI or diabetic conditions. DISCUSSION This study found that the younger patients of SLE, IBD and RA had higher rates of HZ compared to the older patients without AI or diabetic conditions of 60 to 69 years. In general, the risk of HZ associated with AI conditions was approximately 1.5 to 2.0 times higher than the corresponding rates in healthy individuals, and after accounting for age and gender differences among various groups of diseases, the incidence of HZ was more than 3 times higher in some diseases (e.g., SLE) compared to healthy young people. These results suggest that preventive strategies, such as vaccination, could be considered for younger people in high-risk groups, such as LRA or SLE patients. Our results also suggest that the vaccine is comparable in patients with autoimmune disease as it does in a larger population (). Age is the most important risk factor for HZ, and current recommendations on HZ vaccination are based primarily on the age of people. Clinical trials of the zoster vaccine reported that HZ IR in the non-vaccinated group was 10.8/1000 pis for people aged 60 to 69 and 6.7/1000 pis among individuals aged 50 to 59 (), (). By contrast, the annual incidence of HZ is 1,2/1000 pys in healthy adults aged 20 to 40 (–). The live zoster vaccine has been approved for use in the general population of 50 years or more in the United States, but ACIP recommends routine HZ vaccination for people over 60 years of age. Although high rates of HZ have been observed in patients with autoimmune disease at younger ages (, ), few studies have evaluated the spectrum of diseases in adults under 50 years of age or have compared their absolute age to the general population. In addition, concerns have been raised that if patients receive HZ vaccine at the early age, there is insufficient evidence to indicate whether patients need a booster dose no later. The results of our study have shown that the risk of HZ in patients of all ages who have SLE, IBD and RA are comparable and in many cases exceed the risk observed in older people for whom the vaccine is recommended. The reasons for increased HZ risk are probably multifactorial, and increased risk may partly reflect exposure to immunosuppressant drugs (including the use of glucocorticoids), as well as autoimmune conditions themselves. Our findings are consistent with previous studies in the search for a higher risk of HZ associated with increased dose of glucocorticoids () and biological use (). Guidelines for HZ vaccination recommendations for patients with autoimmune disease are not fully compatible with each other. In the United States and Canada, HZ vaccine is considered appropriate for patients who use metotrexate and low to moderate doses of glucocorticoids, but not for patients treated with high-dose glucocorticoids and biologics () (). On the contrary, national committees on immunization practices in Europe recommend avoiding HZ vaccination in patients receiving immunosuppressive treatment (). The European League against Rheumatism recognizes the high burden of HZ disease and recommends HZ vaccination to slightly immunosuppressed patients (). In addition, although the evidence is of very low quality, the recently updated RTA guide conditionally recommended that HZ be given in both early RR patients and established ≥ 50 years (), rather than RR patients ≥ 60 years recommended in guideline ACR 2012 (). These updated guidelines reflect the continuing theoretical concern about vaccine-induced infection associated with a live virus vaccine, especially in patients treated with biology, although the RTA panel considered that AR patients as young as 50 had a sufficient risk for the zoster to consider them appropriate candidates for the vaccine (). Although there are no completed prospective tests that evaluate the clinical safety of the HZ vaccine in large cohorts with AI, a pilot study in 10 patients with SLE did not identify any HZ-related safety problems when patients were vaccinated according to published guidelines. An ongoing trial in inflammatory arthritis patients treated with anti-TNF agents has not revealed any signs of safety(). Our findings suggest that the efficacy of the zoster vaccine for RA patients is comparable to that of patients without AI or diabetic conditions regardless of their age. This finding is consistent with an observational analysis that showed that receiving HZ vaccine was not associated with a short-term increase in the incidence of HZ and in fact, reduction of HZ risk in the long term, in Medicare patients with certain AI conditions, including those exposed to biological (). Preliminary results of the live zoster vaccine have been shown to be safe and effective in HIV-positive patients with CD4 counts ranging from 200 to 350 (). The accumulation of observation or test tests indicates that the routine administration of HZ vaccines for patients with autoimmune disease is well tolerated and has the important effect of reducing the burden of HZ comorbid. In addition, our findings are consistent with previous studies in the search for a higher risk of HZ associated with increased doses of glucocorticoids (, , ).One of the main strengths of this study is that we examine the specific IRHs for age within large cohorts of seven AI diseases and compare these rates to HZ RIs in diabetic and healthy populations. Our study provided HZ IRs for disease and steroid use when patients were in different combinations of these conditions. However, several features of our study design can affect the interpretation of the results. We did not have medical records to confirm the occurrence of HZ, although the definition of HZ using our criterion of an outpatient or outpatient HZ diagnosis has proven to have a high positive predictive value. Sensitivity analysis using an outpatient or outpatient HZ diagnosis accompanied by the use of antiviral drugs presumably gave greater specificity to identify HZ infections and gave similar results in our specific age results. In addition, we found that the HZ IRs in our data were very similar to the HZ rates reported in clinical trials (), () and the erroneous classification of treated HZ was unlikely to be differential by AI condition or drug exposure. In addition, individual medical letters were not used to identify patients with autoimmune diseases; instead, we applied a validated algorithm with more than 80% of PPV. Thus, it is possible to misclassify autoimmune diseases. However, these diagnoses are relatively accurate by requiring all patients in the cohort study who have had at least 2 medical diagnoses and at least the filling or administration of specific disease (–) medicines. Finally, we did not apply a delay time after the vaccine to exclude HZ related to the vaccine, as less than 1% of patients received vaccination during the baseline. CONCLUSION The high rates of absolute HZ incidence in people under 50 years of age with certain autoimmune and inflammatory conditions suggest that it might be appropriate to consider the vaccination of these people on the basis of an absolute risk comparable to the age of healthy people ≥ 60 currently recommended for CDC vaccination. Clinical trials are being conducted on the safety and effectiveness of HZ vaccination among patients with these autoimmune and inflammatory conditions and can provide more definitive information on which to base vaccination decisions and report the need for vaccination, as well as the safety and effectiveness of younger patients with autoimmune and inflammatory conditions. Supplementary Material AppendixS1RecognitionInformations Dr. Curtis received support from the Health Research and Quality Agency (R01 HS018517) and the Acturial Research Corporation (on behalf of the Department of Health and Human Services) Dr. Yun received the support of scholarship 1 K12 HS021694 of the Health Research and Quality Agency, Rockville, MD, USA This work was supported by the Health Research and Quality Agency (R01 HS018517) and the Actuario Research Corporation (on behalf of the Department of Health and Human Services). FootnotesDrs. Yun and Curtis had full access to all the data in the study and were responsible for the integrity of the data and the accuracy of the data analysis. ## ################################################################ ######################################################################################################################################################################################## , 8600 Rockville Pike, Bethesda MD, 20894 USA
The Epidemiology and Prevention of Herpes Zoster | SpringerLink
:max_bytes(150000):strip_icc()/herpes-zoster-0b07f822323246a2ad21057bed5654eb.jpg)
Shingles: Causes and Risk Factors

Varicella Strain Identity in First and Second Herpes Zoster Infection Episodes - Infectious Disease Advisor

What Causes Shingles to Activate and What to Do About It

Shingles - Wikipedia

Shingles: Treatment, Causes, Contagious, Symptoms, Stages & Vaccine

Shingles | Babylon Health

Shingles: Treatment, Causes, Contagious, Symptoms, Stages & Vaccine
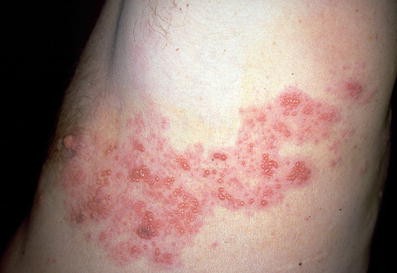
Concomitant Herpes Zoster and Herpes Simplex Infection | MDedge Dermatology
Recurrent Varicella in an Immunocompetent Woman | MDedge Dermatology

Shingles: Treatment, Causes, Contagious, Symptoms, Stages & Vaccine
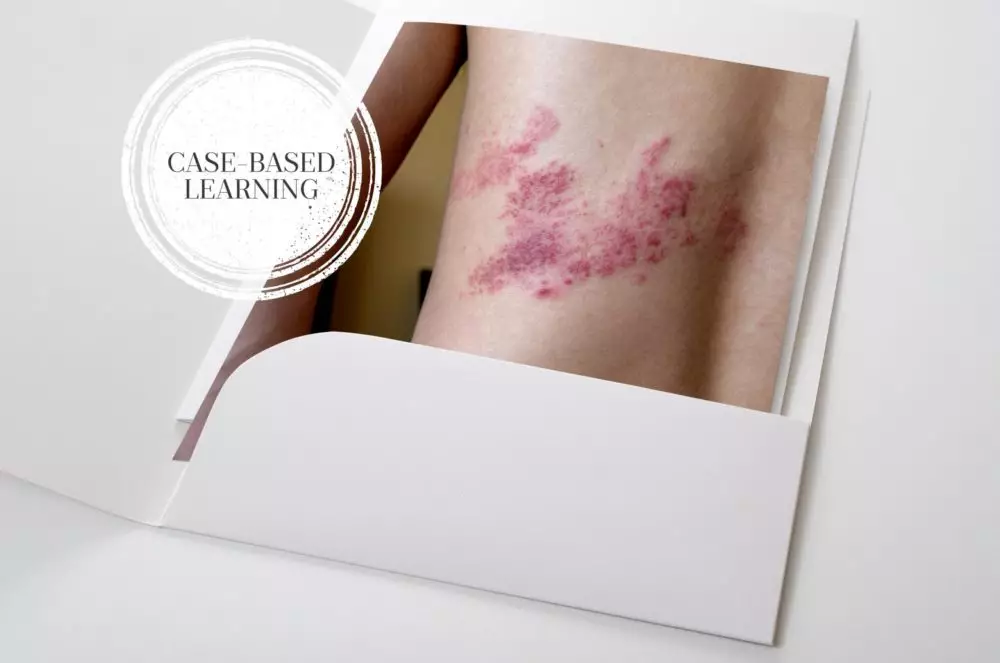
Case-based learning: shingles - The Pharmaceutical Journal

Shingles Recurrence: What You Should Know
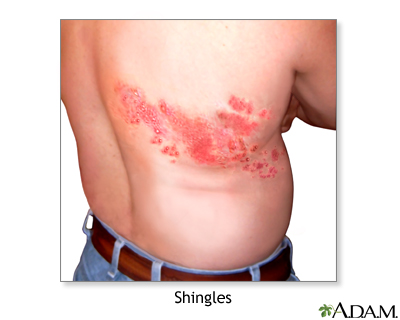
Shingles and chickenpox (Varicella-zoster virus) | Lima Memorial Health System

Shingles: Treatment, Causes, Contagious, Symptoms, Stages & Vaccine

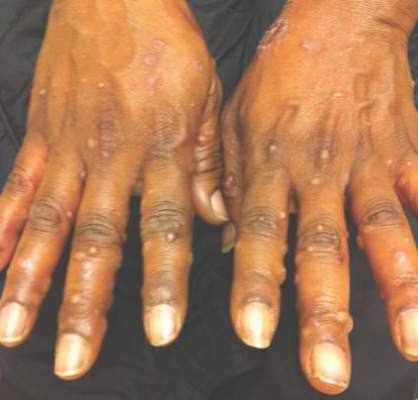
Underlying Diseases in Patients with Recurrent Herpes Zoster a | Download Table
Autoimmune Skin Rash - Drone Fest
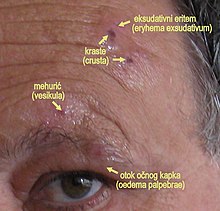
What the Pain of Shingles and Ramsay Hunt Syndrome Feels Like

Shingles Recurrence: What You Should Know
Shingles - Wikipedia

Shingles vs. Psoriasis: What's the Difference?
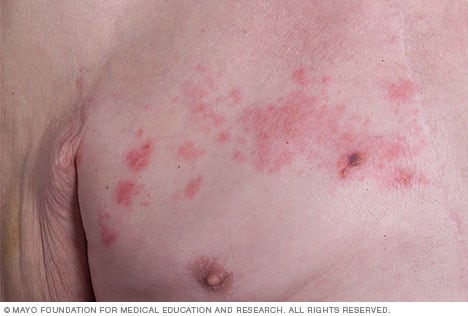
Shingles - Symptoms and causes - Mayo Clinic
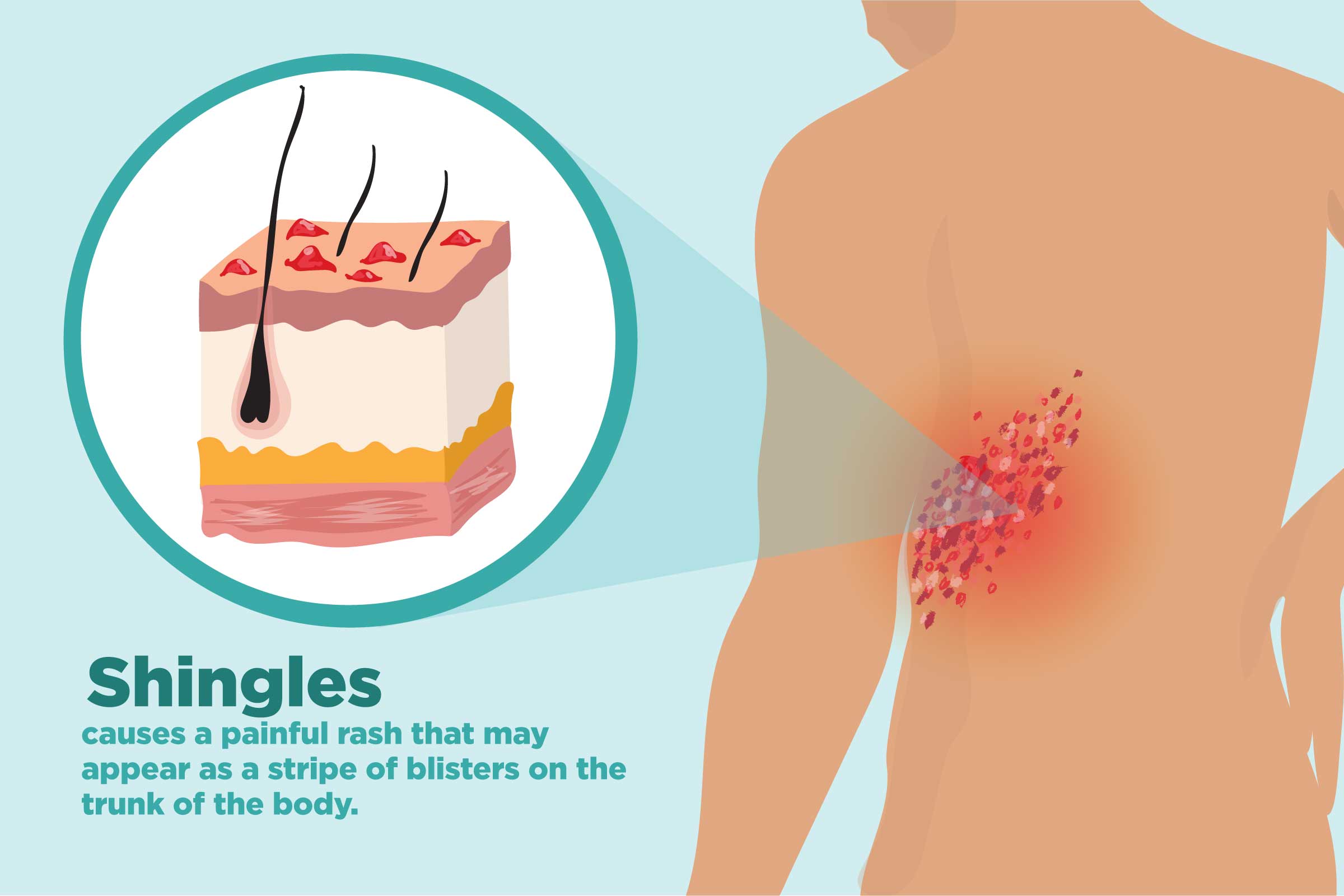
Shingles and Arthritis: 7 Tips to Protect Yourself

Underlying Diseases in Patients with Recurrent Herpes Zoster a | Download Table

31F here we go again. Any else get repeat outbreaks? My daily Valtrex doesn't seem to be helping. : shingles
The stages of shingles and how the condition progresses
Shingles (Varicella-Zoster Virus) | Lorna Vanderhaeghe
/shingles_symptoms_IL-5aec997c8e1b6e0039a1df31.png)
Shingles: Signs, Symptoms, and Complications
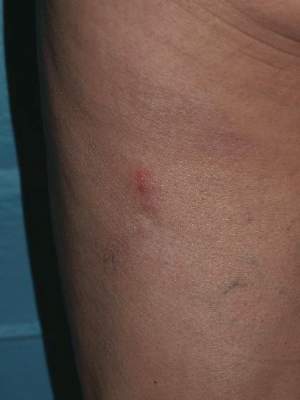
Recurrent Varicella in an Immunocompetent Woman | MDedge Dermatology

The ages at the initial and recurrent episodes of herpes zoster (HZ),... | Download Scientific Diagram

Systematic review of incidence and complications of herpes zoster: towards a global perspective | BMJ Open
Is It Still Shingles If There's No Rash?

Does the Loss of Estrogen From Menopause Up Your Risk for Shingles?
Postherpetic Neuralgia disease: Malacards - Research Articles, Drugs, Genes, Clinical Trials
Shingles Recurrence: What You Should Know
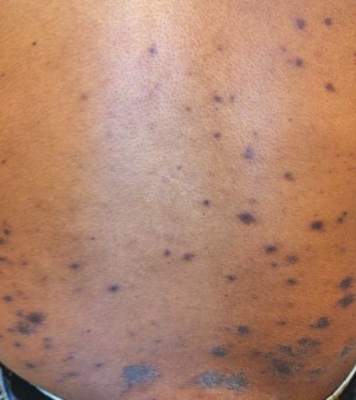
Recurrent Varicella in an Immunocompetent Woman | MDedge Dermatology
/shingles-causes-30-5af4907bc064710036cf2b64.png)
Shingles: Causes and Risk Factors

Shingles: Symptoms, pictures, treatments, and more
Frontiers | Herpes Zoster and Immunogenicity and Safety of Zoster Vaccines in Transplant Patients: A Narrative Review of the Literature | Immunology

Shingles can strike twice. Will the shingles vaccine help? - Harvard Health Blog - Harvard Health Publishing
Posting Komentar untuk "recurrent shingles autoimmune disease"